Catch microbes before they become a problem and work to implement a microbial contamination control strategy. The view of Anne Wagner, PhD, Technology and Market Development Manager for Charles River's Microbial Solutions division.
Manufacturing is often seen and talked about in terms of its efficiency. We've all seen those news reports or commercials showing conveyor belts with bottles filled and packed in seconds. Speed and efficiency are essential to manufacturing, but it all falls apart if it's not based on product quality.
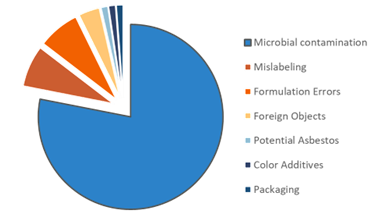
Consider the manufacturing of household and personal care products. These are not "sterile" businesses, but non-sterile does not mean non-quality. One of the biggest antagonists to consumer product safety is microbial contamination. If microbial contamination occurs in your plant, a series of events are triggered to respond, sanitize and remediate the problem, costing time and money. But sometimes the contamination goes undetected and reaches the market, causing a recall with a larger, broader impact. From 2014 to 2019, the vast majority of FDA-requested recalls-78 %-were due to microbial contamination (see illus). Safety Gate, the EU's early warning system, has also detected microbial contamination issues over the years. Not all microorganisms are pathogenic, but products must be free of microorganisms that cause damage.
Notorious culprits behind recent product recalls
Several high-profile incidents of microbial contamination have led to product recalls in recent times. These include Pluralibacter gergoviae and Cronobacter sakazakii. Pluralibacter (Enterobacter) gergoviae is a bacterium commonly found in water. It is classified as an opportunistic pathogen of low risk to healthy people, but can cause infection in people with weakened immune systems. P. gergoviae is of particular interest to the consumer products industry because of its acquired ability to escape common cosmetic preservatives. A publication by M. Periame in the Journal of Applied Microbiology revealed that P. gergoviae has evolved so well that it now has multiple mechanisms, including detoxifying enzymes, flagellin expression, and altered cell membrane structure, to survive in the presence of common cosmetic preservatives. As microorganisms like P. gergoviae become more "intelligent", we must also adapt to keep them out of consumer products.
Another notorious microorganism that has made its way into food and consumer products is Cronobacter sakazakii. Like P. gergoviae, it is also an opportunistic pathogen. In fact, these two microorganisms were previously in the same genus, Enterobacter, but were later classified into separate genera as taxonomy improved. Cronobacter sakazakii was discovered from human clinical isolates and was found to be resistant to desiccation. C. sakazakii has been found in domestic environments and foods. Infections have been reported in elderly and immunocompromised individuals and it is also a high-risk organism for infants when it is found in and contaminates infant formula. In a recall notice issued by the FDA in March 2022, a major producer of powdered infant formula discovered Cronobacter sakazakii in its product and then in its manufacturing facility, resulting in a massive recall and supply chain issue.
Contamination of infant formula with C. sakazakii can put infants at risk for infection, sepsis, meningitis, and necrotizing enterocolitis. 7 Proposed sources of C. sakazakii may be from poor manufacturing practices and contaminated raw materials or from human sources.
Test early and still upstream for microbial contamination
Microbial contamination is not necessarily homogeneous in the product and can have varying rates of growth, so you must be careful not to miss it. Unfortunately, even with final product testing, microbes can go undetected. Testing earlier in the manufacturing process and further upstream to ensure raw material quality and water purity, as well as implementing effective cleaning practices and good manufacturing practices can have a significant positive impact on reducing microbial contamination. These practices are all part of good industrial hygiene designed to find the source of a problem before it gets out of control.
To make a simple analogy, it's like going to the dentist every six months for a regular checkup. By looking early, you can spot a small cavity and fill it before it gets worse. But if you don't spot it and ignore it, that cavity can turn into a painful root canal. Preventive measures can keep small problems from getting bigger.
Recall is the worst-case scenario, but microbial contamination can impact all facets of a business. When a product fails microbial release testing, corrective action is costly, revenue is lost, and manufacturing plants can be shut down, significantly impacting the company's supply chain. In the case of a recall, the same monetary losses occur, but the impact is broader, with very public notifications, and depending on the microbes, the contamination can cause consumer illness and even, in some tragic cases, death. Customers demand quality and it is the manufacturer's responsibility to deliver on that promise of quality. This means catching germs before they become a problem and implementing a microbial contamination control strategy, which means monitoring your environment with modern technology to detect microbial contamination more quickly and accurately. By implementing all of these safeguards, you ensure that your product is of higher quality and has a safety profile that you can guarantee to your customer.