The view of Anne Wagner, PhD, Technology and Market Development Manager for Charles River's Microbial Solutions division.
Environmental monitoring provides the tools necessary to effectively address contamination risks.
The manufacturing of consumer care products is complex and requires the highest level of efficiency and productivity. The process is constantly threatened by unseen invaders: microorganisms. Imagine this scenario. Before a shampoo is released to the market, microbial limits are tested and you discover out-of-specification (OOS) microbial growth on your plates.
What should you do next?
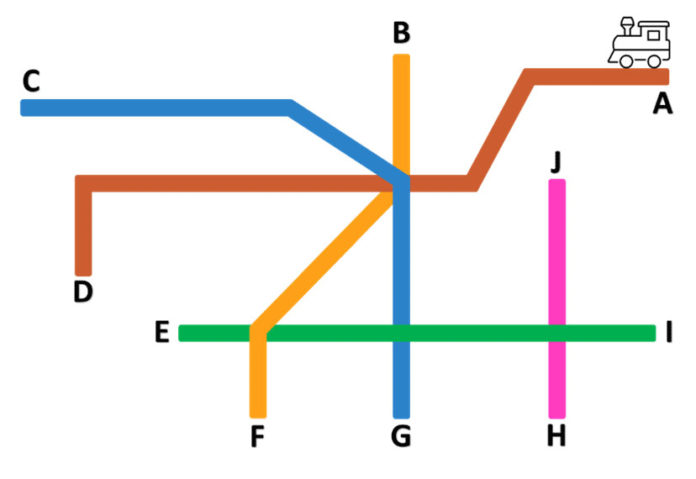
You can imagine your manufacturing facility as a railway system (see illustration). SKU shampoo is produced on the red rail line. Your finished product is complete at stop D.
The first step is to determine which microorganism contaminated your product. You probably performed a Gram stain and then identified the microbial isolate recovered to the species level. You identify the microbe as Pluralibacter gergoviae. This organism is classified as an opportunistic Gram-negative, preservative-resistant pathogen that is ubiquitous in nature. Without a baseline of your environment, each contamination event puts you at square one in identifying the root cause. This microbial contaminant can come from anywhere.
Let's go back to our train analogy. We can see that the contamination occurred on the red line. Think of it as a skid. If you only clean the manufacturing tank, the corrective action may not be enough to fix the problem because there are many other sources of contamination. In addition, the red line route is connected to all other rail lines, directly or indirectly, which means that the risk points for contamination in a final product are expansive. New trains arrive every few minutes and you could see recontamination occur again on the red line if you cannot confirm the original source of the introduction of pathogenic bacteria into the environment.
Was it contaminated raw material, improperly worn PPE or problems with the water purification system? Did construction work or moving vehicles create dust? You need to test all these environmental sources to find the origin of the microorganism. In this example, you determine that the microbial source is at stop J on the pink line. The microbial source could be a leaking pipe, a water purification filter failure, or even an air conditioning problem. No amount of tank cleaning will solve this problem. If the source is a raw material shared between different manufacturing sites, you can potentially have microbial contamination in multiple facilities. The scope of the contamination may be even greater than expected.
Biocontamination tests to the rescue
But hope is not lost! You can regain control of your manufacturing environment through environmental monitoring (EM). EM is a risk assessment control strategy that involves testing for bioburden in your manufacturing environment under the direction of your QC micro-lab. This strategy should be considered for all equipment, personnel, materials, procedures, processes and facilities. Here's how the process works. Locations and materials are sampled and plated on culture media to look for the growth of microorganisms. Colonies of microorganisms are counted, recorded, and the organisms identified. For each location and material, alert and action levels are created from the established baseline amount of manufacturing microbiota. If the number of microorganisms reaches the alert level, it indicates a potential drift from normal operating conditions. If the action level is reached, this indicates a significant drift from normal operating conditions and an investigation should be conducted to understand the reason for this change.
The benefits of environmental monitoring are clear
EM provides you with the tools to quickly assess, mitigate risk and resolve contamination events efficiently and accurately. With action and alert limits in place, even if your manufacturing facility is complex and fast-moving, you can potentially discover contamination before it reaches your final product, protecting customers and saving time and money.
Without the use of EM, the risk of a contamination event is higher. Microbial contamination of a batch cannot be remedied or reversed once it has occurred, and the product must be destroyed. Then the entire train of equipment is shut down, including the batch skid, storage tanks and filling line. You've lost production capacity, revenue and time. You have increased costs and the risk of recall. If the product has been released to the market, these events are often brought to the attention of consumers through publicly announced recalls by regulatory agencies. Product recalls not only pose a risk to consumers, but result in additional costs and lost revenue, create a negative regulatory status and a loss of consumer confidence in the brand.
The goal of a consumer products company is to produce a product that the consumer wants. The product must have all the characteristics of a consumer product - effective, pleasing to the eye and, most importantly, safe for the user. Products must be free of harmful microorganisms that can cause illness or negatively impact the product. Environmental monitoring provides a baseline profile of microorganisms in the manufacturing environment. Implement environmental monitoring with accurate microbial identifications to track microorganisms and trends and manage risks to help your manufacturing site make proactive decisions. The value of environmental monitoring depends on the quality, accuracy and reproducibility of these identifications. Tracking and trending your manufacturing microflora helps you identify areas at risk for contamination and demonstrates that procedures implemented to prevent contamination are effective.